Custom health packaging encompasses a broad range of specialized solutions designed to meet the unique needs of pharmaceuticals, medical devices, nutraceuticals, and other health-related products. It prioritizes safety, compliance, product integrity, and often, user convenience and information clarity.
Types of custom health packaging include child-resistant closures and containers, tamper-evident features, specialized blister packs, custom-designed folding cartons with inserts for vials or syringes, temperature-controlled packaging, sterile barrier systems for medical devices, compliance packaging (e.g., dose-reminder packs), and branded packaging for nutraceuticals or wellness products that require specific protective or communicative features.
The "custom" aspect means that the packaging is tailored to the specific product’s requirements, regulatory landscape, and sometimes, brand identity. While ShineTop’s primary expertise lies in cosmetic packaging, we understand the critical nature of specialized packaging and sometimes produce components like high-quality glass vials or specific plastic containers that can be adapted for health-related products, especially in the wellness and nutraceutical sectors. Let’s explore the diverse world of custom health packaging.
What are the Different Types of Medical Packaging?
Medical packaging is a highly regulated subset of health packaging, specifically designed for pharmaceuticals, medical devices, and diagnostic products. Safety, sterility, and product integrity are paramount.
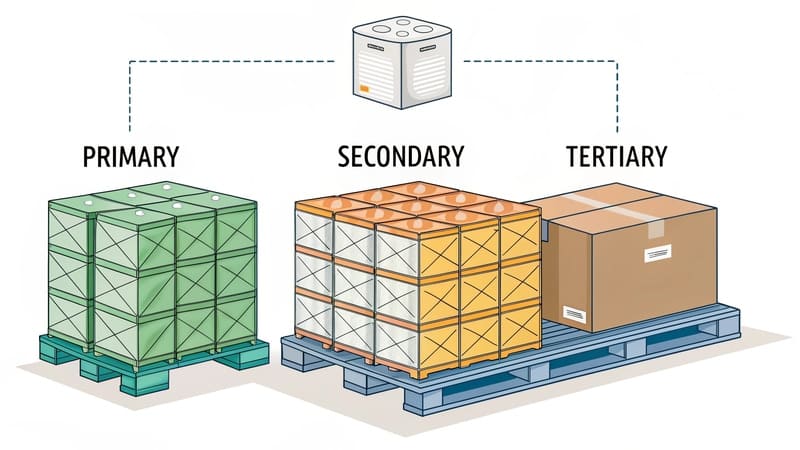
Different types of medical packaging include primary packaging like blister packs, bottles (glass/plastic), vials, ampoules, pre-filled syringes, pouches, and sachets. Secondary packaging includes cartons, trays, and kits. Tertiary packaging involves shippers and insulated containers. Sterile barrier systems are crucial for many medical devices.
Medical packaging must adhere to stringent standards to ensure patient safety and product efficacy.
Common Categories of Medical Packaging:
-
Primary Packaging (Direct Product Contact):
- Blister Packs: Thermoformed plastic cavities with a lidding material (often aluminum foil or paper/foil laminate) for individual doses of tablets, capsules. Can be customized for child-resistance or senior-friendliness.
- Bottles:
- Plastic (HDPE, PET, PP): For solid dosage forms (tablets, capsules) and liquids (oral solutions, suspensions). Often with child-resistant or tamper-evident closures.
- Glass (Type I, II, or III borosilicate or soda-lime): For liquids, powders for injection, parenteral drugs, offering excellent inertness and barrier properties.
- Vials & Ampoules (Glass or Plastic): For injectable drugs, vaccines, biologics. Ampoules are sealed glass containers for single use. Vials can be single or multi-dose, sealed with a rubber stopper and aluminum crimp cap.
- Pre-filled Syringes: Combine container and delivery system, reducing medication errors and improving convenience.
- Pouches & Sachets (Flexible Packaging): Often multi-laminate foils or films for single-dose powders, liquids, transdermal patches, or sterile medical device components.
- Tubes (Aluminum or Plastic): For ointments, creams, gels.
-
Secondary Packaging:
- Folding Cartons (Boxes): House primary containers, provide space for extensive labeling (drug information, dosage, warnings, barcodes, serialization for track-and-trace). Can include custom inserts or dividers.
- Trays: Thermoformed plastic or paperboard trays to hold and organize multiple vials, ampoules, or device components within a kit.
- Kits: Assembled packaging containing multiple components for a specific procedure or treatment.
-
Tertiary Packaging (Transit Packaging):
- Shippers: Corrugated boxes designed to protect products during transit.
- Temperature-Controlled Packaging (Cold Chain): Insulated shippers with coolants (gel packs, dry ice) for temperature-sensitive drugs and biologics.
-
Sterile Barrier Systems (for Medical Devices):
- Specialized packaging (e.g., Tyvek® pouches, thermoformed trays with lidding) that allows for sterilization (e.g., EtO, gamma, steam) and maintains sterility until the point of use.
Customization in medical packaging often involves tailoring these types to specific drug properties (e.g., light sensitivity, oxygen sensitivity), dosage requirements, patient demographics (e.g., senior-friendly features), and regulatory mandates (e.g., serialization for anti-counterfeiting).
What are the Different Types of Packaging?
Understanding the general categories of packaging provides a framework for appreciating where custom health packaging fits and how it adapts these broader types for specialized needs.
The main types (or levels) of packaging are: 1. Primary Packaging (direct product contact), 2. Secondary Packaging (groups primary packs, e.g., retail box), 3. Tertiary Packaging (for bulk shipping), and sometimes 4. Ancillary Packaging (labels, closures, inserts). Each type serves distinct functions in the product lifecycle.
These levels are hierarchical and apply across all industries.
The Packaging Levels:
-
Primary Packaging:
- Definition: The layer immediately surrounding and in direct contact with the product.
- Health Sector Examples: A blister pack holding a tablet, a glass vial containing a vaccine, a sterile pouch enclosing a catheter.
-
Secondary Packaging:
- Definition: Encloses one or more primary packages. Often the unit seen by the consumer in a retail or pharmacy setting.
- Health Sector Examples: The paperboard carton containing a blister-packed medication, the box holding a bottle of cough syrup, a tray organizing several medical device components.
-
Tertiary Packaging (Transit/Transport Packaging):
- Definition: Used to group secondary (or sometimes primary) packages for bulk handling, storage, and distribution.
- Health Sector Examples: Corrugated shipping cartons filled with boxes of medication, insulated shippers for temperature-sensitive biologics palletized for air freight.
-
Ancillary Packaging:
- Definition: Components that support the main packaging functions.
- Health Sector Examples: Patient Information Leaflets (PILs), labels with dosage instructions and warnings, tamper-evident seals, child-resistant closures, desiccant packs, cotton fillers in pill bottles.
Custom health packaging involves tailoring materials, designs, and features at each of these levels to meet the stringent requirements of health products. For instance, a custom feature at the primary level might be a specially coated vial for a sensitive biologic. At the secondary level, it could be a carton with a unique die-cut and insert to hold a pre-filled syringe securely.
What is Primary Packaging of Medical Devices?
Primary packaging for medical devices is exceptionally critical as it often needs to maintain the sterility and functionality of the device until the moment it’s used in a clinical setting or by a patient.
The primary packaging of medical devices is the sterile barrier system or the innermost packaging layer that is in direct contact with the device (or maintains its sterile environment). Its crucial functions are to protect the device from physical damage and contamination, allow for sterilization, maintain sterility until the point of use, and facilitate aseptic presentation.
This layer is fundamental to patient safety and device efficacy.
Key Characteristics & Types of Medical Device Primary Packaging:
-
Sterile Barrier System (SBS):
- The core concept. The SBS is designed to prevent ingress of microorganisms and allow for aseptic presentation of the product at the point of use.
- It must be compatible with the chosen sterilization method (e.g., Ethylene Oxide – EtO, gamma irradiation, steam, e-beam).
-
Common Materials:
- Medical-Grade Papers: Often porous to allow sterilant penetration (e.g., EtO gas) but prevent microbial entry.
- Plastic Films: Polymers like PET, PE, PP, or co-extrusions, chosen for strength, clarity, and barrier properties.
- Tyvek® (Spunbonded HDPE from DuPont): Widely used for its excellent microbial barrier, high strength, puncture resistance, and porosity to sterilizing gases.
- Foils (Aluminum-based laminates): For devices requiring a very high barrier to light, moisture, and oxygen.
- Thermoformable Plastics (e.g., PETG, HIPS): Used to create rigid trays or clamshells.
-
Common Forms:
- Pouches: Pre-formed or form-fill-seal pouches made from combinations of paper/film, film/film, or Tyvek®/film. Often with a peelable seal.
- Bags: Similar to pouches, can be header bags or gusseted.
- Trays with Lidding: Rigid thermoformed trays (often PETG or HIPS) holding the device, sealed with a peelable lid made of coated Tyvek®, medical-grade paper, or foil laminate.
- Blister Packs: Similar to pharmaceutical blisters but designed for devices.
- Vials/Containers (for device components or liquids associated with devices):
-
Essential Features:
- Seal Integrity: Seals must be strong yet allow for clean, easy peeling without fiber tear (for aseptic presentation).
- Peelability: Consistent and clean peel characteristics.
- Puncture Resistance: To protect against sharp device edges.
- Clarity (sometimes): To allow visual inspection of the device.
- Sterilization Indicators: Often printed on the packaging to show it has been exposed to a sterilization process.
Customization involves selecting the right combination of materials and form to match the device’s size, shape, fragility, sterility requirements, and intended use environment.
What is the Packaging of Medical Instruments?
"Medical instruments" can range from simple disposable items to complex surgical tools. Their packaging must ensure they are delivered sterile (if required), undamaged, and ready for use by healthcare professionals.
The packaging of medical instruments, especially those intended for sterile use, involves a sterile barrier system (primary packaging like pouches or trays made of materials like Tyvek®, medical-grade paper, and specific films) designed to allow sterilization and maintain sterility. Secondary packaging (boxes, cartons) provides further protection and organization, often with clear labeling for identification and instructions.
The packaging for medical instruments is all about ensuring safety, sterility, and functionality at the point of care.
Key Aspects of Medical Instrument Packaging:
-
Sterility Maintenance (Primary Packaging – Sterile Barrier System):
- As described above, pouches (Tyvek®/film, paper/film) and rigid trays with peelable lidding are very common.
- The packaging must be compatible with the instrument’s material and the chosen sterilization method (e.g., steam autoclaving for many reusable surgical instruments, EtO or gamma for disposable or heat-sensitive ones).
- For reusable instruments sterilized within a healthcare facility, sterilization wraps (CSR wraps) or rigid sterilization containers are used.
-
Protection from Physical Damage:
- Instruments, especially those with sharp points or delicate mechanisms, need protection from impact, vibration, and compression.
- Custom-fit thermoformed trays, foam inserts, or silicone holders within the primary or secondary packaging can secure instruments.
-
Ease of Identification & Aseptic Presentation:
- Clear labeling on all packaging levels with instrument name, size, type, lot number, expiration date (if applicable), and manufacturer.
- Primary packaging designed for easy, clean peeling to allow for aseptic transfer of the instrument to the sterile field.
-
Organization (for sets or kits):
- Surgical instrument sets are often packaged in custom trays or cassettes (metal or plastic) that organize the instruments for a specific procedure. These trays themselves may then be wrapped or placed in a sterile barrier system.
- Secondary boxes for kits often have custom inserts to keep multiple components organized.
-
Traceability & Compliance:
- Unique Device Identification (UDI) markings are increasingly required on labels and sometimes directly on instruments for tracking.
- Packaging must comply with stringent medical device regulations (e.g., FDA in the US, MDR in Europe).
-
Material Biocompatibility:
- Packaging materials in direct contact with instruments must not leach harmful substances or interact negatively with the instrument material.
Custom health packaging for medical instruments often involves close collaboration between the instrument manufacturer and packaging specialists (like those at ShineTop, if we were producing, for example, custom-designed protective cartons or specialized inserts for instrument kits) to ensure all safety, sterility, and functional requirements are met.
Conclusion
Custom health packaging, encompassing solutions for pharmaceuticals, medical devices, and instruments, is a highly specialized field where safety, sterility, and product integrity are paramount. From child-resistant pharmaceutical bottles and sterile barrier systems for devices to meticulously organized instrument kits, the packaging materials (glass, specialized plastics, aluminum, medical-grade papers) and designs are carefully chosen and customized to meet stringent regulatory standards and ensure the well-being of patients and healthcare professionals.